How to ensure the quality of solid preparations? Preparation technology and equipment give the answer
publisherSteve
time2020/10/29
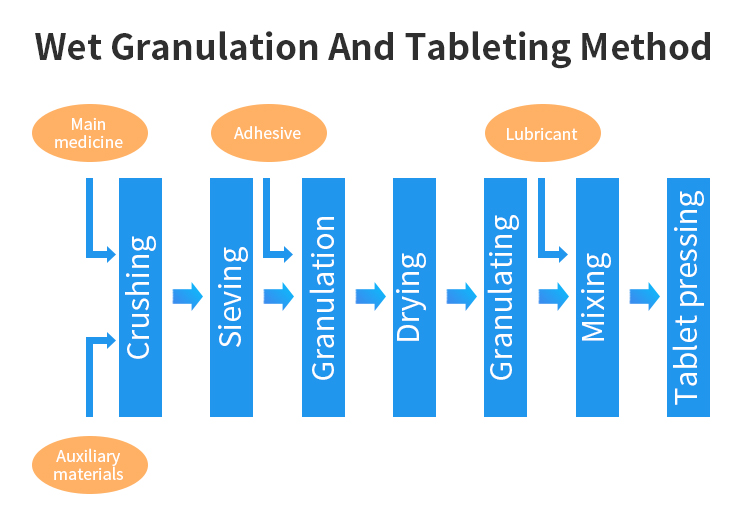
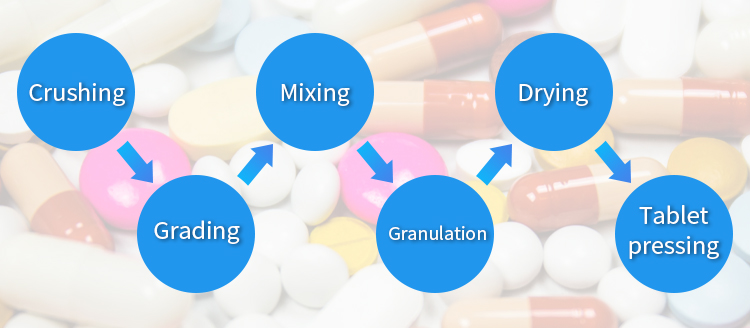
- In pharmaceutical products, solid preparation is a kind of dosage form with wide application and variety, accounting for about 70% to 80%. In order to ensure the product quality and smooth production process of solid preparation, it is often necessary to process and treat the drug, including crushing, grading, mixing, granulation, drying, tablet pressing, etc., and the application of powder technology is permeated in each unit operation.
How to ensure the quality of solid preparations? Preparation technology and equipment give the answer
In pharmaceutical products, solid preparation is a kind of dosage form with wide application and variety, accounting for about 70% to 80%. Since the starting material of solid preparation is powder, in order to ensure the product quality and smooth production process of solid preparation, it is often necessary to process and treat the drug, including crushing, grading, mixing, granulation, drying, tablet pressing, etc., and the application of powder technology is permeated in each unit operation.
It is pointed out in the industry that solid preparation technology and pharmaceutical equipment are the guarantee for the smooth operation of powder and the important tools to ensure the quality of solid preparation products. Therefore, it is very important to select suitable excipients and adopt appropriate preparation technology and equipment.
Taking the preparation process of tablet as an example, it is a kind of tablet solid preparation made by mixing and pressing the drug and suitable excipients evenly. The preparation process of tablet can be divided into three steps: filling, compressing and pushing. In order to ensure the quality of tablets, in the formulation design and excipients screening, the influence of powder properties of tablet materials on tablet formability should be considered, including the analysis of fluidity, compressibility and quantifiable of tablet materials.
With the development of new excipients and efficient tablet pressing equipment, tablet production has entered an era of high efficiency, energy saving and high quality. According to the different properties of the powder, the pelletizing and pressing method or direct pressing method are generally used in tablet pressing. Among them, granulation and tablet pressing methods can be divided into wet granulation and dry granulation.
Specifically, the wet granulation and tablet pressing method has low requirements on the powder properties of raw materials or excipients, which can solve the problem of insufficient powder properties of raw materials and excipients, obtain particles with good fluidity, and significantly improve the compression formability of drugs. However, the disadvantage of wet granulation is that it is not suitable for the drugs with unstable heat and humidity, which may lead to stick impact and top crack in the process of tablet compression.
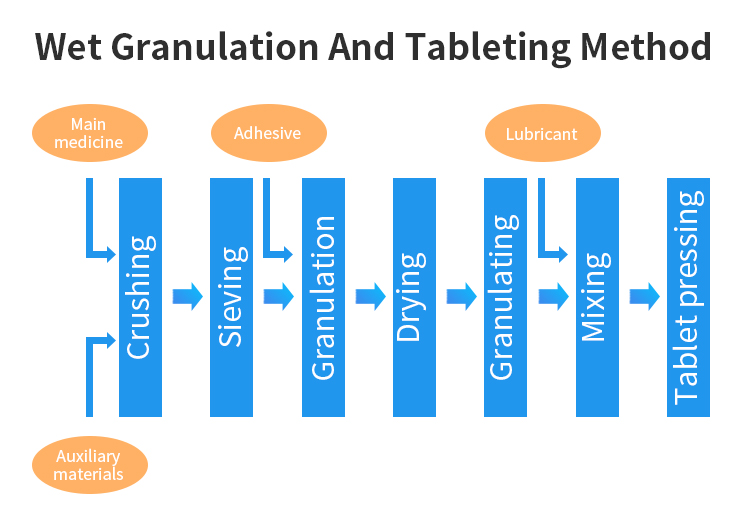
The dry granulation method is a method of crushing and granulating the mixture of raw and auxiliary materials into thin or large pieces. The fluidity and compressibility can also be significantly improved after granulation, which is an effective method for granulation of drugs with unstable damp heat. However, this method has higher requirements on the properties of excipients powder. Excipients should have good plastic deformation, good compression formability or have the function of dry adhesive. Otherwise, it is easy to break into powder when crushing and granulating after tablet pressing, and the yield of particles is not high.
In recent years, with the continuous development of modern science and technology, as well as the promotion of GMP standardization and QBD concept, powder treatment methods continue to penetrate into the preparation process of solid preparations. At the same time, as the solid preparation technology and pharmaceutical equipment directly affect the product quality of solid preparation, it is a trend to upgrade the solid preparation technology and improve the pharmaceutical equipment under the background of the increasingly high requirements of the current pharmaceutical industry for solid preparation products.
From the perspective of the trend, the industry believes that the continuous production mode is the development mode explored and practiced by many solid preparation enterprises at present, that is, from the production of raw materials to the production of products, each process is carried out in sequence according to the process requirements. It is expected that this model will become a development direction of the industry in the next five years or so.
In this context, some manufacturers, such as LTPM CHINA, have actively launched their layout in recent years. For example, some enterprises said that in 2020, the company will focus on the "overall solution supplier of solid preparation intelligent factory" and the strategic layout of big health industry, continue to deeply cultivate the pharmaceutical equipment market and intelligent logistics industry, strive to become the global solution supplier of intelligent pharmaceutical equipment factory, and continue to promote in-depth to the big health industry.
Some enterprises, through years of layout, have promoted the strategy of "one vertical, one horizontal and one platform", constantly enrich their product lines, enhance their competitive advantages in the field of solid preparations, create competitive overall solutions for solid preparations and build pharmaceutical intelligent factories.
